Background:
Despite the introduction of newer drugs and combination therapies, outcomes of patients with AML and TP53mutation (mut) and/or loss continue to remain poor, with a median survival of 6-12 months. The phase 2 study of venetoclax (Ven) added to Clad+ LDAC alternating with azacitidine (Aza) in pts with newly diagnosed (ND) AML demonstrated a composite complete remission (CRc) rate of 93% and median overall survival (OS) not reached (NR) at 22 months (mos) (Kadia et al, JCO, 2022) (NCT03586609) . The trial was amended to increase enrollment, including a prespecified cohort of pts with AML with TP53mut/loss.
Methods:
This is a subgroup analysis of pts with TP53mut/loss on the phase 2 clinical trial. The treatment protocol has been previously published. Pts with AML with TP53 mut (next generation sequencing, lower limit of detection: 2% variant allele frequency [VAF]) or loss by conventional cytogenetics/fluorescent in situ hybridization and/or array comparative genomic hybridization at AML diagnosis were included. CRc was defined as complete remission (CR) plus CR with incomplete count recovery (CRi). Relapse free survival (RFS) was calculated from response to relapse/death and OS from treatment initiation to death; neither censored for allogeneic hematopoietic stem cell transplantation (HSCT).
Results:
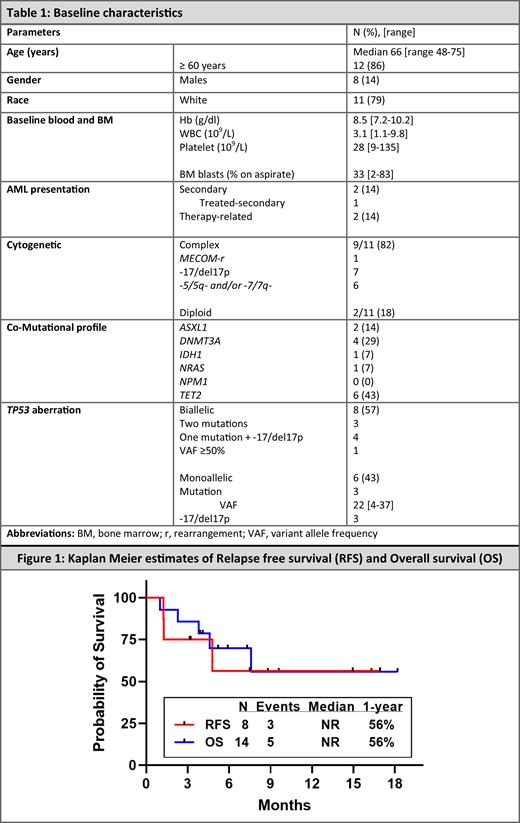
From Nov 2018-Apr 2023, 124 pts with ND AML were treated on this trial, 14 (11%) of whom had TP53mut/loss. The median age in this subgroup was 66 years (yrs) (range 48-75 yrs) and 8 pts (57%) were male. Two pts (14%) had secondary AML (one treated secondary, post HSCT) and another 2 pts (14%) had therapy-related AML. Twelve pts (86%) had a TP53 mut, 8 of whom had bi-allelic loss (two mut, 1 mut with chromosome 17 loss or 1 mut with VAF≥50%). Two pts (14%) had only TP53 deletion (ie. -17/del17p). CRc was attained in 8 pts (57%) [7 CR (50%) and 1 CRi (7%)] and another pt attained partial remission (PR) with 5% bone marrow blasts as best response. 5/8 pts (63%) pts with CRc attained measurable residual disease (MRD) negativity and 4/8 (50%) also had normalization of their TP53 abnormality . The median therapy cycle to best response was 1. Notably 3 pts (21%) had erythroid leukemia, one of whom attained an MRD+ CR. Nine pts had a complex karyotype, 4 (44%) of whom attained a CRc. 4/8 pts (50%) with bi-allelic TP53 loss attained a CRc (2 became MRD negative by flow cytometry and one cleared both TP53 mutations).
Pts received a median of 1 cycle (range, 1-5) of therapy. At a median follow-up of 9 mos from study initiation, the median RFS in the responders was not reached (NR) and 1-yr RFS was 56%; median OS for the entire cohort was NR and 1-yr OS was 56%. The 1 yr-OS in responders was 75%. 4/8 pts with CRc and the pt with PR underwent HSCT at a median of 4.5 mos (range 3.9-5.9 mos) from study therapy initiation; all are alive and in remission at a median of 6 mos (range 1-12.3 mos) from HSCT.
Conclusion:
The phase 2 trial of Ven added to alternating Clad+LDAC and Aza showed promising remission rates of 57% in pts with ND AML having TP53 mut/loss and high rates of MRD negativity in responders; median RFS and OS was not reached at 9 mos follow-up. Five pts (36% overall, and 50% of the responders) could be consolidated with HSCT, with none of them relapsing after HSCT. The trial continues to accrue pts.
Disclosures
Senapati:Kite Pharma: Other: Advisory Board. Loghavi:Gerson Lehrman Group: Consultancy; QualWorld: Consultancy; Caris Diagnostics: Consultancy; Blueprint Medicine: Consultancy; Abbvie: Consultancy; Guidepoint: Consultancy; Recordati/ EUSA Pharma: Consultancy; Daiichi Sankyo: Consultancy; Amgen: Research Funding; Astellas: Research Funding; Abbvie: Current equity holder in publicly-traded company. Daver:Hanmi: Research Funding; Amgen: Consultancy, Research Funding; Novimmune: Research Funding; Celgene: Consultancy; AROG: Consultancy; Astellas: Consultancy, Research Funding; Jazz: Consultancy; Trovagene: Research Funding; Trillium: Consultancy, Research Funding; Agios: Consultancy; Gilead: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; FATE: Research Funding; AbbVie: Consultancy, Research Funding; Syndax: Consultancy; Novartis: Consultancy; Glycomimetics: Research Funding; Shattuck Labs: Consultancy; ImmunoGen: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Servier: Consultancy, Research Funding; Kite, a Gilead company: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Kronos Bio: Research Funding. Borthakur:Astex Pharmaceuticals, Ryvu, PTC Therapeutics: Research Funding; Pacylex, Novartis, Cytomx, Bio Ascend:: Membership on an entity's Board of Directors or advisory committees; Catamaran Bio, Abbvie, PPD Development, Protagonist Therapeutics, Janssen: Consultancy. DiNardo:Novartis: Honoraria; Notable Labs: Honoraria; AbbVie/Genentech: Honoraria; Astellas: Honoraria; ImmuniOnc: Honoraria; Servier: Honoraria; Fogham: Honoraria; Takeda: Honoraria; BMS: Honoraria; Schrödinger: Consultancy. Jabbour:Hikma Pharmaceuticals: Consultancy, Honoraria, Research Funding; Ascentage Pharma Group: Consultancy, Honoraria, Research Funding; Bristol-Myers Squibb: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Genentech: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Adaptive Biotech: Consultancy, Honoraria, Research Funding. Short:Takeda: Consultancy, Research Funding; Astellas: Research Funding; Amgen: Honoraria; AstraZeneca: Consultancy; Stemline therapeutics: Research Funding; Pfizer: Consultancy; Novartis: Consultancy. Pemmaraju:Physician Education Resource (PER): Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ASH Committee on Communications: Other: Leadership; ASCO Cancer.Net Editorial Board: Other: Leadership; Karger Publishers: Other: Licenses; United States Department of Defense (DOD): Research Funding; National Institute of Health/National Cancer Institute (NIH/NCI): Research Funding; Patient Power: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; PeerView Institute for Medical Education: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Dava Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Harborside Press: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Intellisphere: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Imedex: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Magdalen Medical Publishing: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Medscape: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Menarini Group: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; OncLive: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CareDx: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CancerNet: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Aplastic Anemia & MDS International Foundation: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Dan's House of Hope: Membership on an entity's Board of Directors or advisory committees; Pacylex: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Neopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; EUSA Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Curio Science: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Stemline: Consultancy, Membership on an entity's Board of Directors or advisory committees; PharmaEssentia: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Protagonist Therapeutics, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Cimeio Therapeutics AG: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ImmunoGen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ClearView Healthcare Partners: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CTI BioPharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Blueprint: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Aptitude Health: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; HemOnc Times/Oncology Times: Other: Uncompensated; Bristol Myers Squibb Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Maiti:Lin BioScience: Research Funding; Celgene: Research Funding. Issa:Celgene: Research Funding; Kura Oncology: Consultancy, Research Funding; Syndax: Research Funding; Novartis: Consultancy, Research Funding; NuProbe: Consultancy; Merck: Research Funding. Shpall:Celaid Therapeutics: Membership on an entity's Board of Directors or advisory committees; Navan: Membership on an entity's Board of Directors or advisory committees; Takeda: Other: License agreement; Axio: Membership on an entity's Board of Directors or advisory committees; Fibrobiologics: Membership on an entity's Board of Directors or advisory committees; NY Blood Center: Membership on an entity's Board of Directors or advisory committees; Adaptimmune: Membership on an entity's Board of Directors or advisory committees; Affimed: Other: License agreement; Syena: Other: License agreement. Garcia-Manero:Genentech: Research Funding; Bristol Myers Squibb: Other: Medical writing support, Research Funding; AbbVie: Research Funding. Ravandi:Prelude: Research Funding; Xencor: Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Biomea fusion: Honoraria, Research Funding; Syros: Consultancy, Honoraria, Research Funding; Celgene/BMS: Consultancy, Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Astellas: Consultancy, Honoraria, Research Funding; Astex/taiho: Membership on an entity's Board of Directors or advisory committees, Research Funding. Kadia:AstraZeneca: Research Funding; Genzyme: Honoraria; Cellenkos Inc.: Research Funding; Cyclacel: Research Funding; Astellas Pharma Global Development: Research Funding; Cure: Speakers Bureau; Biologix, Cure, Hikma Pharmaceuticals: Speakers Bureau; Jazz Pharmaceuticals, Pfizer, Pulmotect, Inc, Regeneron Pharmaceuticals, SELLAS Life Sciences Group: Research Funding; AbbVie, Amgen, Inc, Ascentage Pharma Group, Astellas Pharma Global Development, Astex, AstraZeneca, BMS, Celgene, Cellenkos Inc, Cyclacel, Delta-Fly Pharma, Inc, Genentech, Inc., Genfleet, Glycomimetics, Iterion, Janssen Research and Development: Research Funding; Ascentage Pharma Group: Research Funding; Genentech: Consultancy, Research Funding; Janssen Research and Development: Research Funding; Hikma Pharmaceuticals: Speakers Bureau; Amgen, Inc.: Research Funding; Glycomimetics: Research Funding; Iterion: Research Funding; Celgene: Research Funding; Delta-Fly Pharma, Inc.: Research Funding; GenFleet Therapeutics: Research Funding; Daiichi Sankyo, Genentech, Inc., Genzyme, Jazz Pharmaceuticals, Liberum, Novartis, Pfizer, PinotBio, Inc, Pulmotect, Inc, Sanofi-Aventis, Servier: Consultancy; Agios: Consultancy; Servier: Consultancy; Liberum: Consultancy; Novartis: Consultancy; Pfizer: Consultancy, Research Funding; Pulmotect, Inc.: Consultancy, Research Funding; Regeneron Pharmaceuticals: Research Funding; Sanofi-Aventis: Consultancy; SELLAS Life Sciences Group: Research Funding; Pinotb-Bio: Consultancy; BMS: Consultancy, Research Funding; Astex: Honoraria.